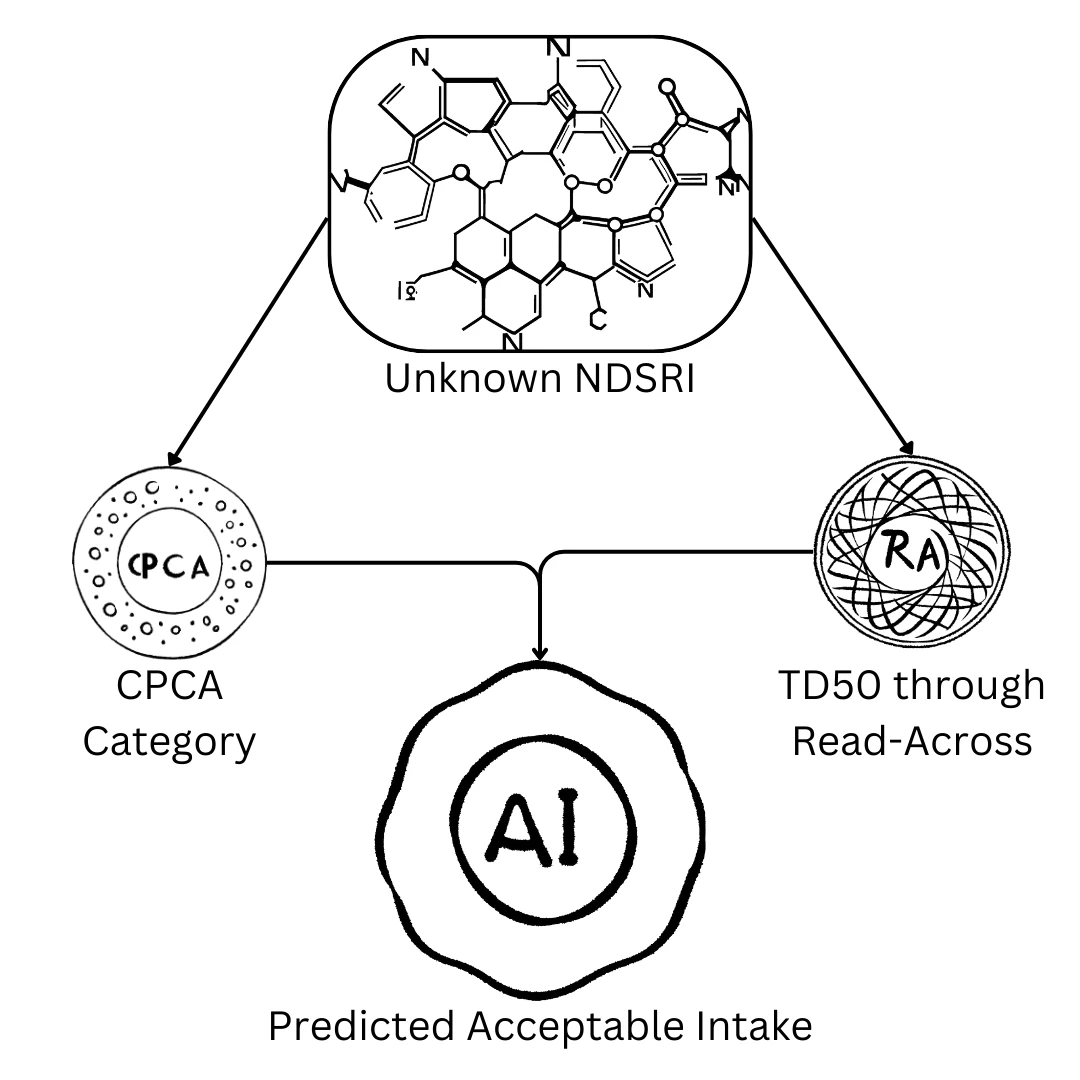
Address the challenge of Nitrosamines. Our specialised Nitrosamines service provides a robust and comprehensive approach to identifying and managing nitrosamine risks in your pharmaceutical products. We specialise in assessing unknown NDSRIs (Nitrosamine Drug Substance-Related Impurities) that can be challenging to identify through traditional methods.
By utilising powerful predictive technology like TD50 through Read-Across, we can bridge toxicological data gaps, allowing us to predict the acceptable intake (AI) and categorise your compounds according to regulatory guidelines. This service provides you with the critical data and documentation required for confident regulatory submissions, helping you navigate this complex and evolving area of pharmaceutical safety.
Identification of Nitrosamine Risks
- Focus on NDSRIs (Nitrosamine Drug Substance-Related Impurities) that evade traditional detection methods.
- Advanced Computational Screening to predict possible nitrosamine formation.
- Early Risk Flagging to prevent late-stage development delays.
Regulatory Confidence & Submission Support
We provide the evidence and documentation you need for successful regulatory approval.
- Comprehensive Justification Reports to support AI levels and risk assessments.
- Alignment with Global Guidelines (ICH M7, EMA, FDA).
- Expert Guidance for navigating the evolving regulatory landscape on nitrosamines.
Predictive Toxicology & Exposure Limits
Our in-silico tools and read-across strategies help close critical data gaps in nitrosamine safety assessment.
TD50 Read-Across Modelling
Acceptable Intake (AI) Prediction
Define safe exposure limits in line with global standards.
Carcinogenicity Potential Categorization Approach
Classify compounds according to regulatory frameworks (EMA, FDA, ICH).

