Optimise your formulations from the start. Our in-silico approach to Formulation Development helps you anticipate and address impurity-related challenges long before they become a problem. We use our advanced predictive technology to analyse the APIs, excipients, and process conditions of your formulation. This allows us to determine what potential impurities can form, how they are likely to form, their likelihood of forming, and their possible toxicity.
By providing these critical insights, we help you make informed decisions during your early development phase. Our analysis allows you to confidently select the safest ingredients and processes, significantly reducing the need for extensive physical testing and accelerating your time to market.
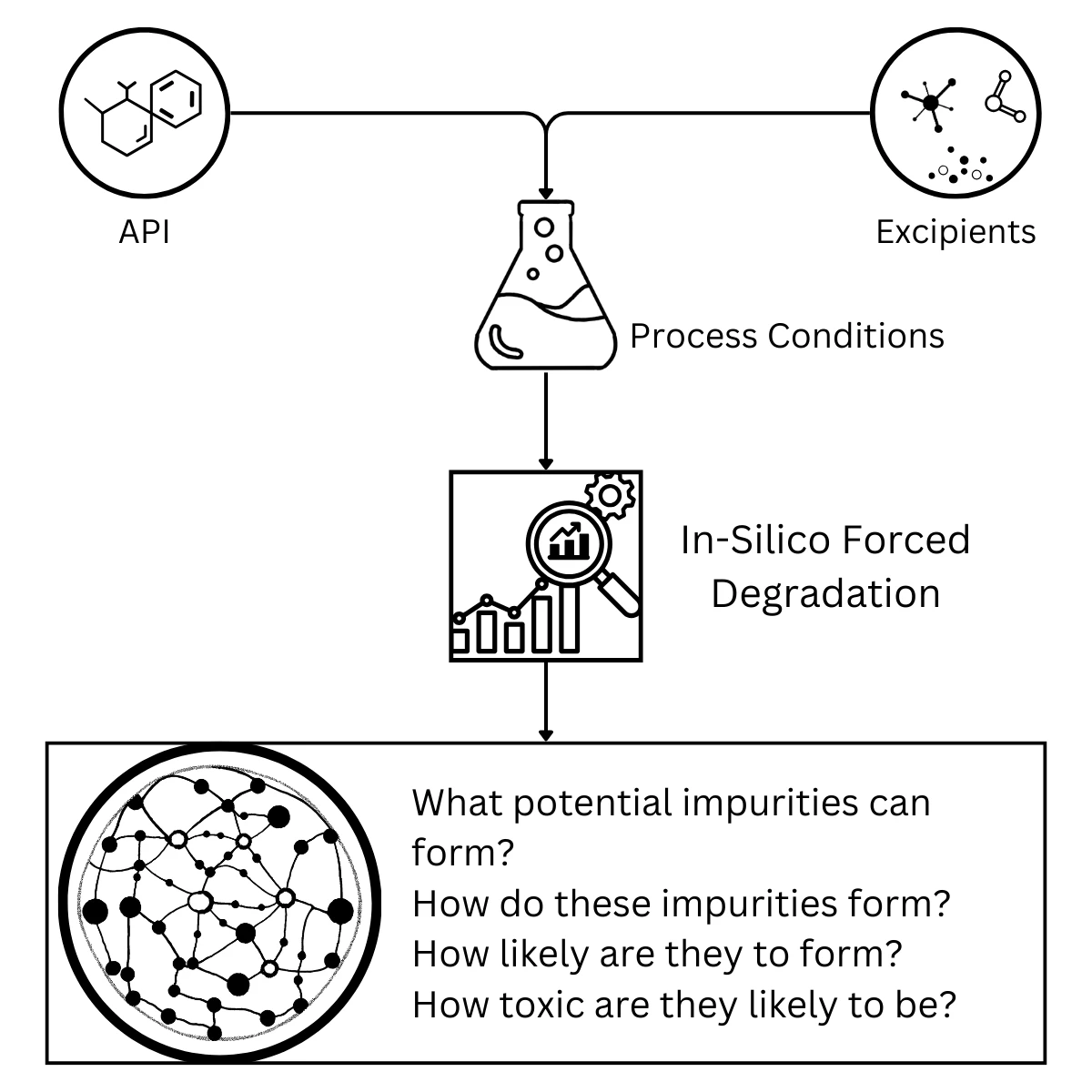
Predicting Impurity Formation in Formulations
We apply computational models and predictive toxicology tools to analyse the chemical interactions within your formulation.
- API–Excipient Interactions: Anticipate potential incompatibilities and degradation pathways.
- Drug–Drug Interactions: Evaluate combined therapies for impurity formation risks.
- Process Condition Analysis: Understand how manufacturing and storage may influence impurity profiles.
Early-Stage Risk
Insights
Our predictive approach gives you the foresight to manage impurity risks before they impact development.
- Impurity Formation Pathways: Identify how impurities are likely to form and their probability.
- Toxicity Prediction: Assess the potential health risks of predicted impurities.
- Safer Ingredient Selection: Choose excipients and APIs with lower risk profiles for long-term safety.
Accelerating Development & Reducing Costs
Reduced Physical Testing
Faster Time to Market
Accelerate development with data-driven formulation choices.
Regulatory Confidence
Build strong safety evidence for smoother submissions.
