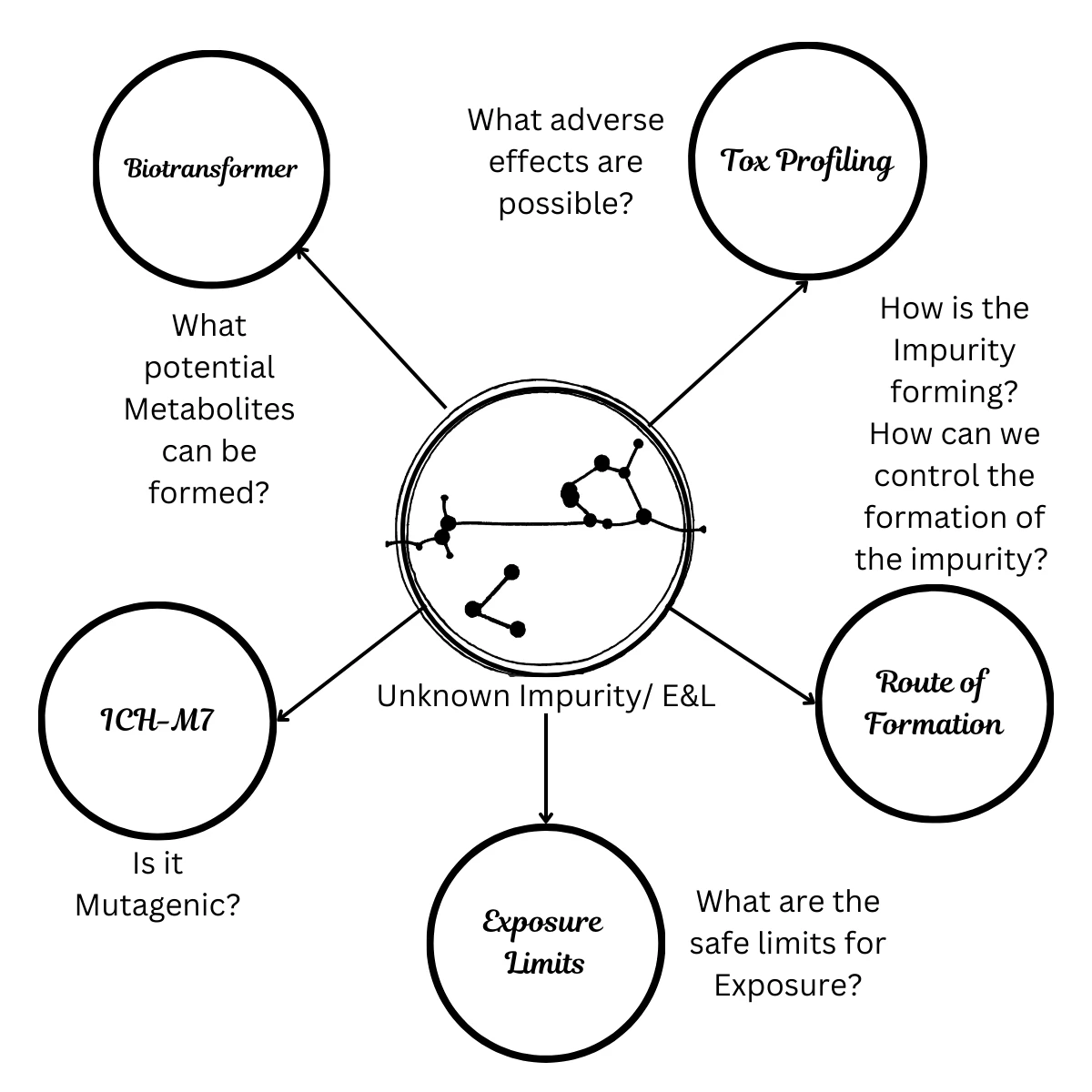
Manage risk with confidence. The Impurity Management service provides a comprehensive and proactive assessment of potential impurities and their adverse effects. We utilise a suite of advanced tools, including Biotransformer and ICH-M7 assessments, to determine if a substance is mutagenic and to understand how impurities form. This in-depth analysis enables us to provide you with a comprehensive Route of Formation report, allowing you to control the formation of impurities in your compounds precisely.
We also go further by establishing safe exposure limits (PDE, OEL, AI) for impurities, extractables, and leachables. By providing a clear Toxicological Assessment and expert guidance, we give you the data needed for confident regulatory submissions and the assurance of a safe, high-quality product.
Identification & Prediction of Impurities
We assess and predict impurities using a suite of advanced in silico and regulatory tools.
- Biotransformer & ICH-M7 Assessments: Evaluate mutagenic potential and metabolic pathways.
- Route of Formation Analysis: Understand how impurities are generated during synthesis or storage.
- Structural Evaluation: Identify potential risks at the earliest stages of product development.
Establishing Safe Exposure Limits
Our toxicology experts determine acceptable exposure levels, ensuring patient and operator safety.
- Health-Based Exposure Limits: PDE (Permitted Daily Exposure), OEL (Occupational Exposure Limit), and AI (Acceptable Intake).
- Extractables & Leachables: Assessment of material-derived impurities in packaging and medical devices.
- Risk Characterisation: Clear interpretation of toxicological data for regulatory acceptance.
Regulatory-Ready Toxicological Assessment
Comprehensive Toxicological Assessment
Regulatory Confidence
Data packaged for ICH, EMA, FDA, and global compliance frameworks.
